The atoms. Any mystery you will
ever hear is nothing in front of the mystery of the atoms. No one has ever
understood what these things are and how do they work at the quantum level.
Speaking of quantum mechanics, let's assume that you already do not have any
idea about what this quantum mechanics is. We'll talk about the quantum when we
know how the atom works. Why is an atom so mysterious? We all have been taught
the basic structure of an atom. But before we jump into that, let us first
understand what an atom is and let us explore and dive deeper into the ocean of
the atomic world. And yes, extra warning, read the article fully, because I am
sure, it will change your way of thinking of life that you see.
Let us not ask what an atom is. Let the atom ask what we are. Yes. What are we? What are you exactly? It is so weird to say 'what' instead of 'who' when we say this phrase. But come on, let us not understand this in the English sense, but let us understand atoms in the chemistry sense. Let us answer what are we instead of who are we. If you think about it, we are nothing but giants of organ systems packed together nicely in a system under a skin. Now we also know what is matter after all, we have explained it like trillions of times to you. And we all know everything that we see around 'is' matter and not 'made up' of matter. Why did I say that? Because there is a huge difference in saying something which is made up of something and on the other hand, is that something itself. Now I know you are very very confused at this point, but no problem, let us proceed. We also know that matter around us is made up of atoms. Now that doesn't mean everything in the universe is matter and that, they are made up of atoms. No. Although you can safely say that everything 'around' you is matter but you can not say everything in the universe is matter. There exists other forms of substances also known as dark matter, anti matter, exotic matter etc. Okay but now, what are atoms! We are said to be made of tissues and cells. Have you wondered what these tissues and cells are made of? Well, if you take what's called an electron microscope, you will find something crazy: atoms! Yes, the minutest substance of any matter is known as atom. The smallest group of particles that constitute matter are the atoms. Atoms. Small word, big meaning. Small in size, has made the biggest effects in the human era. If you take matter, cut it down, cut it down and cut until you can't cut it anymore. If it would have been possible, you could've shrunk yourself down and cut it to the point where you could not cut it anymore. That point is the atom. Now a couple of paragraphs later, I will tell you that this is not true in one sense, but yeah just for simplicity, let us assume what Enlightened Wisdom: Smart Science is telling you, is correct (at least for now). So we now have some idea about what are atoms. What we'll be focusing on next is the analysis of an atom or the structure of an atom.
So, in terms of microbiology, we see that atoms are invisible to naked eye. They are so small that it cannot even be seen with a microscope. You would need an electron microscope to see atoms. Atoms as we all know are the smallest substances that make up everything in the universe, matter as well as antimatter. Observe carefully, atoms are a group of some other substances which are known to be the smallest thing to be seen in any object. Atoms were first discovered by John Dalton in 1808. But he didn't really say much about the subatomic particles which are the particles that make up the atom. This is where you will know that atom is not the smallest particle of matter. So atoms as we know are the smallest particle of a chemical element that can exist. When we talk about the structure of an atom, we need to know what is a charge first. Because atoms, having a size of 0.1 nanometers, do not care of the gravitational force. The big guy gravity doesn't has much to do with atoms. It is almost negligible at this small scale. Does that mean atoms play with no force? Well, there is a special force for these minute particles. It is known as the electrostatic force. Humans play with gravity and atoms play with electrostatics. Wait, what does 'play' here mean, literally? Play here means that there exists that particular type of fundamental force between the two objects whether they are humans or atoms. Confused? See as we all know, between any two objects/body, there exists gravitational force between them. And gravity is an attractive force. Then why don't two humans stick to each other? It is because the force of gravity between the two body is so less that it is almost negligible to say that there is any attraction, although there is a little. And when we talk about atoms, at that scale, gravity doesn't matter much. What really do matters, is the electrostatic force. What is that? Well, there exists a phenomena called charge. An electric charge is the property of an atom where the atom either has more electrons or has more protons, which creates a difference in the overall net charge of an atom, thus the atom has a 'charge'. Just like gravity, electrostatic force also exists between two atoms causing them to either repel or to attract. When will they repel or attract? Have you ever heard of magnets? We all know there are two poles of a magnet. A north pole and a south pole. Or a positive side and a negative side. The positive side in physics is represented as a '+' sign and the negative side in physics is represented as a '-' sign. We all also know that like charges or like 'sides' repel or go against each other and unlike charges or opposite 'side' attract or go stick to each other. And now, having this knowledge, you are ready to know the structure of an atom. So what does an atom look like basically? We all imagine atoms as little circular things where there is a circular nucleus and there are circular shells in which there are again circular balls which we call electrons and these things revolve around the nucleus. I mean first, this is so boring to read at first, and second I have told you the same thing trillions of times in my post of the atoms which I made in 2021. I am not saying that the info was wrong. But the thing is, if you happen to believe in something what's called quantum mechanics, you'll understand that atoms do not look like this. Then what do atoms look like exactly? This is why I said that atoms are the most mysterious things in the universe. Let us see what we can do next, to understand atoms.
If you have read my previous post on atoms, you may know that an atom is made of three different types of particles. The electrons, the protons and the neutrons. Okay. So before entering the quantum aspect of atoms, let us first understand the structure of an atom. An atom, for beginners has a big nucleus (the atom is very small, but the nucleus of the atom makes 99% of the atom, thus nucleus of an atom is large when talking about the structure of an atom although it is very very small) which contains of two different types of particles. Protons and neutrons. Protons were discovered by Ernest Rutherford and Goldstein in the early 1900's. Protons have a positive charge and neutrons have, well, guess it. Neutrons were discovered by James Chadwick in May 1932. Okay, so neutrons have no charge which means they are neutral. No charge nothing. They're just particles. Then why do we even need neutrons in an atom? It is because neutrons exert a special force called the nuclear force which holds up protons in its nucleus. Now let us understand it why. Let's imagine an atom having 2 protons and 2 neutrons, let's just forget about the electrons at least for now. As we know, like charges repel and unlike charges attract. Now why does this happens is very interesting, but that's the thing for some other day. For now you should know that protons have a positive charge in them. And when there are protons having the same like charge in a nucleus, they must repel, don't they? Then how are they stuck to each other as there must be a large large electrostatic force between them. This is where neutrons come to the rescue. Neutrons take the burden to hold 2 or more protons together. How? By nuclear forces. Now by this, you must've got the idea that there are basically 4 fundamental forces in the universe.
1) gravity, 2) electrostatic or electromagnetic force, 3) strong nuclear force and 4) weak nuclear force. Read more on the four fundamental forces in the universe (physics): The four fundamental forces in the universe. Okay, so now we know about the two particles in the atom: the protons and the neutrons. Yeah, if you want to learn more about them, I highly recommend you to read this article on protons and neutrons: The atoms: electrons, protons and neutrons. So now, let us know what these electrons are as these are something for which all atoms go crazy for. These are the particles that can move here and there. Leave a kingdom, join another. Destroy, live, whatever is there, is only because of the electrons.
Let us start learning about the most valuable particle in the universe. The electron. Now why is the electron so valuable? First let's understand what an electron is, an electron is an elementary particle! What? It was supposed to be a subatomic particle, wasn't it? Well, as I told you, 99% of an atom is the nucleus. This shows how small is the mass of an electron. Thus it is considered as an elementary particle. We'll not go too deeper into the topic of elementary particles today, but yes, elementary particles are those type of particles that are even smaller than subatomic particles like proton and neutron. So you see, you were first told that the atoms are the smallest things in the universe. Then you got to know that "No no, subatomic particles must be the smallest things in the universe". But sadly, that too didn't last any longer. Because now, you know that elementary particles for example quarks which are a type of particles that make up the protons and neutrons itself, are one of the smallest particles in the world. And yes this is the correct answer. Quarks are the particles that make up the particles present inside the nucleus i.e. the proton(s) and the neutrons. So now, we all see in the atomic modals and diagrams, electrons are revolving around the nucleus of an atom. Now this makes a lot of sense when you try to understand and learn chemical science. Electrons were first discovered by JJ Thompson in October 1897. It was Thompson who first said that atoms contained a negatively charged particle as well. The electrons. Now usually, atoms have the same number of electrons as their are the number of protons. Thus cancelling all the positives and the negatives which create a neutral charge and thus atoms are considered to be neutral. This is the reason why we don't see electrostatic force around us much. But yes, if there is any charge difference, there is an electrostatic force working at our level as well. For example: when you rub comb or a ruler with your hair, you will observe that when brought near to pieces of paper, these things get attracted towards them! Now let us understand why an electron is so important in an atom.
The electrons aren't stationary. They are said to be moving continuously around the nucleus of an atom. Now the electrons are said to be revolving in shells of an atom. The circular path in which electrons are revolving are called shells or energy levels of an atom. This means that in an electron shell, you can either remove or add electrons! Yes. Let us see some reasons why an electron is amazing.
1) In a metal element, the electrons of the atom can jump off to a higher orbit around the nucleus. As it does this, it absorbs some energy. And when the electron falls back to its original orbit, it shoots out the light energy as a Photon. This is the reason why metals have the physical property of luster. You can read more about this in my previous post: What is quantum mechanics?
2) Electrons are elementary particles, which means they have very
very small mass. Way too smaller than a proton or neutron. Now electron is an
example of a lepton, which is also an elementary particle. I have explained
elementary particles just before. If you want to know more about them, read
this article: The theory of the standard model of particle physics.
So now, what is special about elementary particles? Elementary particles are the smallest known building blocks of the universe. They are thought to have no internal structure, meaning that researchers think about them as zero dimensional points that take up no space.
3) The most important thing about electrons is that they are the reason for electricity! Flow of electrons gives us electricity which then, we use it as a powerful resource to live our life! Electrons play a vital role in our lives. Electrostatics and electricity are some of its features.
4) Electrons are also used for making bonds or sharing atoms
itself! Meaning, an electron can connect to atoms or remove itself from its
original atom and join another atom's shell! Now, when we talk about bonds,
there are 2 popular types of bonds that we mostly hear: ionic bonds and covalent
bonds. Some real life applications? Salt that you eat is a
result of a bond formation of two different elements sodium and chlorine.
Carbohydrates that we eat, is also a result of a bond formation of atoms.
Now see, there are many more valid reasons as to why is an electron so useful. But for now, these should be enough. And because of that many atoms are kind of like in a war to either take electrons or give electrons or share electrons or borrow electrons. They have their own little kingdom.
I told you that there are two popular types of bonds, ionic and covalent.
Keep in mind that there are a lot more types of chemical bonds out there. But
let us just understand these two, as these are the types of bonds that
are the most important to know and are very useful.
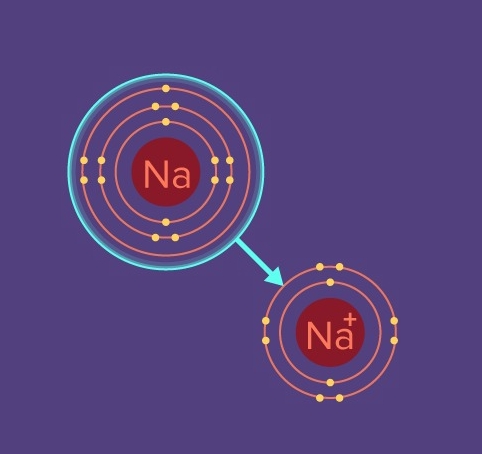
These are a type of bonds where an atom gives its electron to another element's atom. Let's say, an atom wants to get rid of an electron and another atom seriously needs an electron. Now why does an atom wants to get rid of an electron badly and why does the other wants an electron badly is a very good question that you can ask. Atoms like to be neutral. Now why do they want to be neutral, we don't know that exactly. But we do know that everything in the universe wants to be stable. Now the question arises. How can something be stable? Simply, by just making an equilibrium. Hey, big word! What is equilibrium? Just the state where the quantities are equal in number and in the case of atoms, they will be stable only when they have the equal number of protons and equal number of electrons. But that was the special case only made for noble gases. What are noble gases? They are special elements present in the periodic table of elements which have a valency of 0. Now let us come back to ionic bonds. Let us say, that sodium which is an element, has only 1 electron in its valence shell or the outermost shell. I mean, sodium which has 11 protons, 11 neutrons and 11 electrons, has 3 shells for its electrons to live in. The first shell has a vacancy for 2 electrons. So, there goes our 2 electrons. Now we have 9 electrons left. As per the octet rule the second shell can have maximum 8 electrons. So there goes the other 8 electrons as well. Now we have to move to the next shell, the third shell and now we have only 1 electron left. So automatically, the third shell of the sodium atom will have only 1 electron. Sodium can be stable only when it's shells are fulfilled, its shells should contain all the required electrons. To do that it either needs to remove that 1 electron in the third shell so that the first and second shell are already completed and there will be no third shell or add another 7 electrons to complete the third shell thereby obeying the octet rule. Now what is the octet rule? The octet rule states that shells after the first shell can hold maximum 8 electrons. So now, you tell, is it easier to give away 1 electron or try to get 7 more electrons? It is so obvious that giving away 1 electron requires way lesser energy for sodium to be stable and become a positive ion. This energy which is required to make an atom an ion is known as the ionization energy. Therefore sodium will have more positive charge as an electron with negative charge will be removed thus the sodium will become an ion and it will be stable. So this was all about stability. Now we will discuss ways in which several atoms can be stable. Let us start with ionic bonds first.
Ionic bonds are created when an atom gives away its valence electron to another atom to attain stability. Like sodium and chlorine. Sodium is an alkali metal and chlorine is a halogen. These are all classifications of elements in the Mendeleev's periodic table. We have created articles that might help you in chemistry. Now, as sodium is an alkali, it has 1 valence electron. Now what is this valency we're keep talking about? In short, a valence electron is nothing but the electron present in the outermost orbit or shell of an atom. And sodium has only 1 of them. And to attain stability, sodium really wants to give away its 1 free electron to somebody so that it gets rid of the third shell and gets satisfied and stabilized with the completely filled first and second shells. Now if sodium really wants to give his electron, then whom shall he give it to? Here comes chlorine, where as it is a halogen, it requires 1 electron to become stable. Halogens are a group of elements which have 7 electrons in their outermost shell. Which means halogens seriously need just 1 electron to become a negative ion or just attain stability. So one wants badly and the other wants to give away badly. So what do they do? They form an ionic bond! Sodium, when exposed to chlorine, will give away its valence electron and thus become a positive ion whereas chlorine gains the electron sodium gave to it and thus becomes a negative ion. Both of them become stable. Happy world. Now ions exert electrostatic force on each other, which forms ionic bonds. Now why is sodium a positive ion and why is chlorine a negative ion, might have been guessed by you till now. If not, then the basic idea is, as an electron gets removed from sodium, the net charge of the sodium atom no longer remains 0. It had 11 protons exerting let's say 11 positive charge. When it had 11 electrons. It has 11 negative charge as well. So 11 positive got cancelled out by 11 negative thus 11-11= 0 net charge of the atom. Now once the valence electron is removed, there are only 10 electrons left which exert 10 negative charge. Now it cancels only 10 positive charge but at last, 1 positive charge remains as originally there were 11 positive charges only. Now 11-10= 1 positive charge of the atom. Now charges aren't measured like these. This was just to provide an idea as to why sodium became positive after losing an electron. And now you know, it is because sodium has a extra positive charge after losing an electron thus sodium becomes a positive ion. And now simple idea as to why chlorine becomes negative. It had 17 electrons and after ionic bonding, got 1 extra from sodium thus creating 18 electrons. Earlier it had 17 electrons and 17 protons thus net charge is again 0. But once it gains an electron, the net charge becomes negative. As +17-18= -1. Here "-" means negative which shows that chlorine, after getting an extra negative electron has extra negative charge. Thus it is very simple to comprehend that chlorine after ionic bonding becomes a negative ion.
Other examples of ionic bonds are: lithium fluoride, lithium
bromide, lithium iodide, lithium chloride, sodium iodide etc. Now the beauty
is, you will understand each and every example above all by yourself, once you
understand the periodic table. For now, let us
move to the covalent bonds.
Covalent
bonds occur when sharing of electrons exist between the atoms but in the case
of sodium chloride, the sodium atom completely transfers the electron to the
chlorine atom, and hence we knew it was an ionic bond. So when does a covalent
bond happen to occur. Let us understand it more deeply. Now by definition,
covalent bonds consists of mutual sharing of one or more pairs of electrons
between two atoms. Now let us understand it. Unlike ionic bonds, there is no
sharing of electrons completely in covalent bonds. The electrons were getting
transferred in ionic bonds, but here, the whole shell gets shared with another
atom in covalent bonds. Interesting, isn't it? Lets simplify. When the bond
formed between 2 atoms is by sharing the electrons in order to attain their
nearest noble gas configuration, then the bond is to be known as the covalent
bond. A chemical bond formed by the sharing of an electron pair between 2 atoms
so that both the atoms get their octets complete, is called as a covalent bond.
The molecules formed as a result of sharing of electron between 2 atoms are
called covalent bonds. I hope it is clear to you now.
The atomic models you have been studying till now are widely accepted in the world due to their simplicity. What if I tell you that the real face of an atom is rarely even taught at schools. The quantum mechanical model of an atom shows us the real picture of atoms which is a lot different than the atomic models we've seen so far. The quantum mechanical model of atom describes the 3-D position of electrons in a probabilistic manner with the help of mathematical calculations. A famous scientist named Erwin Schrödinger made this model. Now, before we proceed, we must know that everything in quantum realm is about waves and their wavefunctions. Therefore any particle in our world is represented by a wave in the quantum world. This property is also known as wave particle duality. To determine the position of electron in an atomic wave, we first take the amplitude of the wave and then square it. We get a new wave. This new wave helps us determine the probabilistic positions of electrons in an atom.






.gif)


.gif)

Nice
ReplyDeleteThank you.
DeletePost a Comment